Intermec Medical Wristbands
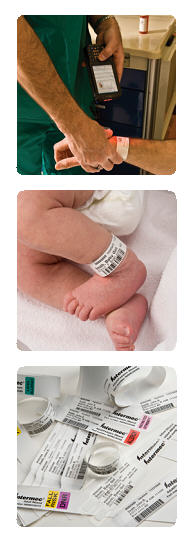 |
Effective patient care is predicated on accuracy, but human error is exceptionally difficult to eliminate in the inherently high-touch medical environment. To address this, barcodes and automatic ID technologies are being used increasingly across a wide range of medical practices. Patient wristbands are one common application area. Intermec patient wristbands ensure positive identification for integration with verification systems for medication, samples, and surgical procedures. The five rights of medication safety (right patient, right drug, right time, right dose, right route) provide an important framework for verification of pharmaceutical administration, but commonly rely on manual human checks. Integration of positive patient ID with barcode wristbands provides a highly reliable adjunct to eliminate human error. With increasing implementation of patient electronic medical record (EMR), scanning the wristband can trigger caregiver notification of allergies or other special requirements. Intermec INband wristbands provide the positive identification hospitals need for maximum patient safety. Excellent print quality ensures both standard and 2D bar codes will be scanned accurately. Durable topcoating provides high resistance to soaps, hand sanitizers, moisture, and other conditions commonly encountered in the hospital environment. Flexible and lightweight, the Intermec band is easy to apply, minimizing the curl that may be experienced with other products. High tensile strength film and a high-shear adhesive closure securely affix the band in place for the duration of the patient stay. A robust tamper-evident closure feature protects the integrity of the patient identification system. Direct thermal technology delivers mission-critical printing system reliability, while providing straightforward compliance with regulations for security of patient information. Simple, intuitive printer loading and one-at-a-time printing allows bands to be generated with minimal waste. Designed to accommodate a wide range of needs, Intermec INband configurations are available to identify patients from newborns to adults. With safety and quality of care depending on accurate patient identification, wristband system components must work together flawlessly. Intermec printers and media are optimized to deliver superior performance when used together. Our rigorous testing and co-engineering ensures consistently high print quality, proven performance in demanding real-world environments, and maximum print head service life for reduced downtime. Benefits:
|
|---|
| Specifications | |
|---|---|
| Size Adult: Small Adult/Pediatric: Infant: | 28.6mm x 292mm 25.4mm x 279.4mm 25.4 x 206.4mm Overall (19mm ID Section with 10mm Strap) |
| Wristband Material | INband/Infant INband with Direct Thermal Print Layer |
| Colour | White |
| Durability | Excellent resistance to hand sanitizer, soap, water and other common exposures |
| Optional HF RFID Specifications | |
| Operating Frequency | 13.56MHz (HF), ISO15693-3 |
| EEPROM | 2048 bit (256 byte), 64 4 byte blockd |
| Unique Identification Number (UID | 64 bit (8 bytes) |
| Data Retention | 10 Years |
| Write Endurance | 100,000 cycles |
| Read/Write Range | 89mm (Typical) |
| Optional Colour Condition Label Addition Specifications | |
| Size | 21.6mm x 26.2mm |
| Material | Tamper Evident Syntran |
| Durability | Excellent resistance to hand sanitizer, soap, water and other common exposures |
| Colours | Purple - DNR Yellow - Fall Risk Red - Alergy |
| Part Number | Model Number | Description | RRP Inc GST |
T % |
|---|---|---|---|---|
| [A] Intermec INband Medical Wristbands | ||||
| INBND26275 | ||||
| INBND26568 | ||||
| INBND27556 | ||||
| [B] Intermec INband Medical Wristbands with RFID | ||||
| ILR00257 | ||||
| ILR00257/4 | ||||
| Intermec PC23 Wristband Printer | ||||

